Covidien achieves CE Mark for Nellcor SpO2 patient monitoring system
27 November 2014
Covidien has announced EU approval for its Nellcor Bedside SpO2 patient monitoring system, PM100N.
The system features home care and sleep study modes that comply with standards for medical electrical equipment used in the home health care environment.
It features continuous monitoring of blood oxygenation (SpO2) and pulse rate, along with patient trend data to help clinicians detect and respond to dangerous respiratory events sooner. With Covidien-exclusive digital signal processing, the PM100N offers speed and accuracy, and reliable pulse oximetry readings, even during low patient perfusion, motion and other forms of signal interference.
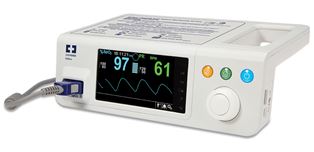
The pulse oximetry system incorporates unique SatSeconds alarm management technology to help distinguish between serious and minor events, reducing clinically insignificant desaturation alarms. The monitor can also be password-protected to prevent lay users from inadvertently modifying settings.
“Just because a patient is discharged from the hospital does not mean his or her care ends,” said Dr. Scott Kelley, chief medical officer, Respiratory and Monitoring Solutions, Covidien. “The continuation of safe, quality care for patients in all settings, including the home, is something we are focused on at Covidien. This new system includes all of the leading Nellcor technology that clinicians have trusted for more than 30 years, with additional features that make it well suited to the home healthcare environment.”
Source: Covidien