Plasmonic biosensors make highly sensitive diagnostic devices
8 July 2014
A new type of highly-sensitive and low-cost sensor, called a plasmonic biosensor, could detect a range of biomarkers that diagnose diseases at an early stage.
Biomarkers, which are chemicals such as proteins in blood or saliva, for example, whose presence or abnormal concentration is caused by a disease. Biomarkers can indicate the presence of diseases long before the appearance of symptoms. However, currently the detection of these molecules still requires specialised laboratories and is costly.
The EU-funded research project called NANOANTENNA, which completed in March 2013, developed a plasmonic nanobiosensor for the detection of proteins. It consists of nanoantennas, tiny gold rods about 100 to 200 nanometres long and 60 to 80 nm wide. By shining light onto such a nanoantenna, the electrons inside start oscillating, amplifying the light radiation in hot spots on the antenna, explains Pietro Giuseppe Gucciardi, a physicist at the Institute for Chemical-Physical Processes, affiliated with the Italian National Research Council CNR, in Messina, Sicily.
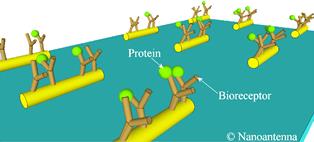
Diagram showing nanoscale gold rods (nanoantennas)
with bioreceptors and proteins (biomarkers).
During the 1990s’ researchers found that plasmons, tiny waves of electrons in metallic surfaces that appear when such surfaces are illuminated, also amplify the light in an area close to that surface. In biosensors, protein molecules are identified by irradiating them with infrared light and by analysing the spectrum of the light they emit, known as a Raman spectrum. If these molecules are close to nanoparticles, the plasmons in the nanoparticles enhance the Raman signal coming from the protein molecules by several orders of magnitude.
The nanoantennas developed in this project only enhance the emitted Raman signal if the biomolecules are close to the hot spots. Therefore, the molecules have to be trapped to be detected. To do so, the researchers attached fragments of DNA engineered to recognise specific proteins, termed bioreceptors, to the nanoantennas. When these nanoantennas with bioreceptors are incubated in a solution that contains the biomarkers, the biomarkers become attached to the nanoantennas. When, subsequently, these nanoantennas are illuminated with light, they show the Raman fingerprints of both the bioreceptor and the biomarker.
"It is important to fund this research because it will be a component of future medicine," says Alexandre Brolo, professor of chemistry specialised in nanotechnology research, who has been developing plasmonic biosensors at the University of Victoria, British Columbia, Canada. He also believes that such approach will make medical care more cost effective. "You want something that is very cheap and is not going to put a big burden on the healthcare system," says Brolo.
"Small, compact and autonomous devices with the same features in terms of sensitivity and robustness as current commercial instrumentation based on plasmonics are still needed," says Maria Carmen Estévez, a researcher at the Catalan Institute of Nanoscience and Nanotechnology in Bellaterra, Spain. The "end-users" of these biosensors have to understand that the development of these devices by researchers in many disciplines is a long process, notes Estévez. She adds that these biosensors will need to be integrated with optical components, with electronics for reading out the measurements, software to process all data, and rely on the use of microfluidics to prepare and process the sample.
Youris.com asked Pietro Giuseppe Gucciardi about the kind of improvements this new technology could bring to biosensors:
Do the molecules to be detected have to be trapped by these nanorods?
Yes, we make the nanoantennas functional by attaching bioreceptors to their surfaces. We use fragments of DNA, so-called haptomers, which are engineered to recognise and trap specific proteins, such as manganese superoxide dismutase (MnSOD), a biomarker for several types of cancers. The nanoantennas are incubated with a solution containing biomarker molecules that become attached to the bioreceptors on the nanoantennas. When they are illuminated with light, we get the vibrational fingerprints of both the bioreceptors and the attached biomarkers.
As a proof of concept, we incubated the functionalised nanoantennas with MnSOD, and with BSA (bovine serum albumin, which does not attach to the bioreceptors). We found that we could only detect vibrational fingerprints of MnSOD.
What is the advantage of using such approach compared to other testing methods?
Typically, kits that are now available are based on fluorescence and you have a sensitivity going to extremely small scale, down to one nanomolar, or even one picomolar. But the real advantage is that with the nanoantennas we can reach a sensitivity of a femtomolar. And what is fascinating is that we even have proof of concept that you can detect single molecules by their vibrational fingerprint. The other advantage of this approach is that the detection method, Raman spectroscopy, does not require staining of the target molecules, it is label free.
Are biosensor kits using nanoantennas now available for clinical
tests?
No, our project finished in March 2013. And our aim was to
deliver a proof of concept. We will first have to implement the
technology in a microfluidic circuit, a biochip, in which we can
test biofluids, such as saliva or blood. This biochip should be
combined with a spectrometer that should be portable. For the moment
we have a table-top spectrometer linked to a computer. We should aim
at developing a suitcase spectrometer based on optical fibres.
Source: youris.com