New microscopy technique rapidly detects skin cancer tissue during surgery
28 January 2014
The US National Institute of Biomedical Imaging and Bioengineering has developed a microscopic technique to analyze and remove cancerous tissue rapidly in the operating room, removing the need for lengthy repeated tissue sampling and checking.
The technique, called strip mosaicing confocal microscopy, can dramatically reduce the length, inefficiency, and expense of this procedure.
The common surgery for non-melanoma skin cancer, known as Mohs surgery, typically achieves excellent results but can be a long process, as the surgeon successively removes the tissue of concern until the surrounding tissue is free of cancer. To determine whether further tissue removal is necessary, the borders of the lesion must be processed in a laboratory to check for residual cancer tissue, a process that takes 20-45 minutes and is often repeated numerous times.
It can take one to three hours, or even longer depending on the size and location of the lesion. The process is lengthy because after a section of tissue is removed, it must be frozen and stained so it can be examined to ensure the borders are clear of residual tumour. Although highly effective, the current practice is labour intensive for surgeons and assisting staff, as well as lengthy and stressful for patients. The time spent by surgical personnel and those analyzing the tissue in the lab increases the expense of the procedure, which has been estimated to cost US$2-3 billion per year in the US.
NIBIB-supported researchers led by Dr Milind Rajadhyaksha at Memorial Sloan Kettering are using their expertise in optical imaging to improve this common procedure. Optical imaging is a technique that uses visible or near-infrared light to obtain detailed images of organs, tissues, and cells. The investigators developed a new pathological assessment technique using a type of optical imaging called strip mosaicing confocal microscopy. It can provide high resolution images during removal of basal cell and squamous cell carcinomas (non-melanoma skin cancers) and perhaps other tumours of the skin.
The new technique uses a focused laser line that performs multiple scans of the tissue to obtain image strips that are then combined, like a mosaic, into a complete image of the excised tissue. The process takes only 90 seconds and eliminates the need to freeze and stain the tissue samples for analysis — a process that takes 20-45 minutes.
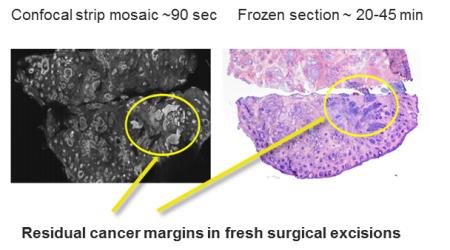
Comparison of residual cancer detected with the new confocal imaging technique and the currently used freezing and staining technique. Source: Dr Milind Rajadyhyaksha, Memorial Sloan-Kettering Cancer Center.
The technique was tested on 17 patients with 34 tissue samples. The overall image quality was excellent, with high resolution and contrast, providing for good visibility of the epidermis and dermis. Researchers compared the new technique against the Mohs approach with its frozen section processing. The new technique achieved a promising 94% in preliminary measures of sensitivity and specificity for detecting skin cancer margins, which is comparable to the 'gold standard' Mohs procedure. These preliminary results demonstrated that the optical technique could potentially detect skin cancer margins with the same accuracy as the conventional frozen section technique.
The results of this study were obtained under laboratory conditions; a clinical trial is now being conducted to demonstrate the feasibility of using this technique in the clinical setting, the ultimate goal of the research group.
Steve Krosnick MD, NIBIB director for the Program for Image-Guided Interventions, explains the utility of the optical system: “The technology is particularly well-suited for Mohs-trained surgeons, who are experts at performing excisions and interpreting images of tissue samples removed during the Mohs procedure. Image quality, ability to make accurate interpretations, and time savings will be key parameters for adoption of the system in the clinical setting, and the current results are very encouraging.”
Reference
1. Larson B, et al. Detection of skin cancer margins in Mohs excisions with high-speed strip mosaicing confocal microscopy: a feasibility study (pages 922–926). British Journal of Dermatology, Vol 169, issue 4, Oct-2013/ R01 EB012466