Microvisk introduces world’s first MEMS-based diagnostics system in the US
31 August 2011
North Wales-based Microvisk Technologies has introduced its handheld devices that monitor the blood clotting status of patients to the US market using MEMS technology on a disposable strip.
The devices branded as ‘CoagMax’ and ‘CoagLite’ are a point-of-care test and a home-use test respectively that clinicians and patients can use to establish the correct dosage of anti-coagulation medication such as Warfarin and to monitor treatment. The devices are set to be trialled with 250 patients in three major cardiac centres in Florida from October with product launches scheduled for mid-2012.
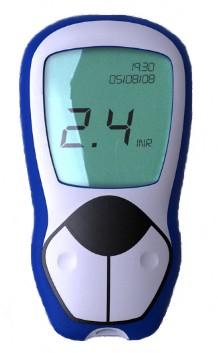 Both
devices incorporate a disposable SmartStrip that uses embedded
sensors to measure the clotting speed of blood from a drop of the
patient’s blood taken by a finger prick, with the results displayed
on a handheld reader. SmartStrip is the world’s first medical
diagnostic strip to be based on a micro-electro-mechanical system
(MEMS) with an on-board memory chip and was originally created as a
movement system for nano-robots.
Both
devices incorporate a disposable SmartStrip that uses embedded
sensors to measure the clotting speed of blood from a drop of the
patient’s blood taken by a finger prick, with the results displayed
on a handheld reader. SmartStrip is the world’s first medical
diagnostic strip to be based on a micro-electro-mechanical system
(MEMS) with an on-board memory chip and was originally created as a
movement system for nano-robots.
MEMS technology is used in the computer projector, iPhone and Nintendo Wii as well other technology based applications. Existing devices deploy optical analysis or measure chemical reactions, requiring a patient to provide more blood and producing a less accurate and less robust result.
Multicentre European clinical trials of CoagMax and CoagLite are already well under way in the UK and Germany. The devices will be introduced to the German market in November at the Medica trade fair.
John Curtis, chief executive officer of Microvisk, said: “We are delighted at the overwhelming interest shown by US distributors and potential partners in our CoagMax and CoagLite devices — it is extremely encouraging. We remain on track to commence US clinical trials this autumn and are gearing up for product launch there in summer 2012.
“We are also continuing to make excellent progress with patient trials in the UK and Germany. We have been expanding our UK manufacturing facilities and are recruiting additional staff as we prepare for European product launches in early 2012.”
Microvisk has established a US operation at Florida and recently appointed medical industry veteran, Bill Moffitt, as company chairman. Mr Moffitt has over 30 years experience in the diagnostics and medical device industry and is the president and CEO of Nanosphere in the US and the chairman of Glysure in the UK.
Bill Moffitt said: “The reaction to our introduction of the CoagMax and CoagLite devices to the US market was all that we could have wished for. These innovative devices will transform the way that blood testing is carried out and Microvisk now has the opportunity to capture a substantial share of the market for doctor’s office and home tests for Warfarin patients.”
Seven million people in the western world use Warfarin and the Food and Drug Administration (FDA) estimates that over one million new patients start taking the drug every year. Patients must have regular blood tests at their doctor’s surgery or hospital clinic to ensure they receive the correct dose. Warfarin is affected by food and exercise and if the dose is too low there is a risk of blood clots forming which can result in a stroke or heart attack, while too high a dose can lead to a life threatening bleed.
The Microvisk devices enable patients to test their blood clotting ability at home, in the same way that people with diabetes test for glucose.
John Curtis added: “Our significant market opportunity in the US and Germany is driven by their healthcare systems, which have introduced payments to all at-risk Warfarin users to do weekly home blood tests, rather than having to go to the doctor or hospital clinic.”
To date, only three companies have developed a test system for blood coagulation that can be used in a doctor’s surgery and although certified for home use, market research shows that doctors feel that they are insufficiently robust and too complex for home use.
The Microvisk SmartStrip is unique in the blood clotting diagnostic world as a solid state system that is robust and simple to use at home. It also requires far less blood than other systems, which means less pain for the user. The coagulation status (clotting speed) of the patient is measured by tiny multi-layered paddles on the surface of the strip and a memory chip ensures the device is calibrated to provide the highest levels of accuracy, while the MEMS technology means that high volumes of the device can be manufactured at low cost.