Antibody injection could limit inflammation damage from heart attacks and strokes
3 May 2011
A simple injection of an antibody could limit the devastating inflammatory response following restoration of blood supply after heart attacks and strokes.
An international team identified an enzyme, MASP-2 (mannan binding lectin-associated serine protease-2), that is found in blood and is a key component of the lectin pathway of complement activation, a component of the innate immune system.
The lectin pathway is responsible for the potentially devastating inflammatory tissue response that can occur when any bodily tissue or organ is reconnected to blood supply following ischaemia — a temporary loss of that blood supply and the oxygen that it carries. This excessive inflammatory response is, in part, responsible for the morbidity and mortality associated with myocardial infarction (heart attack) and cerebrovascular accidents (CVAs or strokes).
The research team succeeded in finding a way to neutralise this enzyme by raising a therapeutic antibody against it. A single antibody injection in animals has been shown to be sufficient to disrupt the molecular process that leads to tissue and organ destruction following ischaemic events, resulting in significantly less damage and markedly improved outcomes.
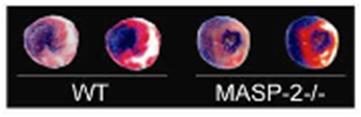
Differences in infarct sizes (white areas of
heart sections) between mice of the same litter that have MASP-2 in
their blood (WT) and those that don’t (MASP-2 -/-). In absence of
MASP-2, the damage to the heart caused by transient loss of blood
supply is smaller.
The research has been published in the Early Online Edition of the Proceedings of the National Academy of Science (PNAS) [1]. Professor Wilhelm Schwaeble of the Department of Infection, Immunity and Inflammation at the University of Leicester, initiated and co-ordinated research collaborations with King’s College London, the Medical University of Fukushima, Japan and the State University of New York.
“This is a fascinating new achievement in the search for novel treatments to significantly reduce the tissue damage and impaired organ function that occur following ischaemia in widespread and serious conditions such as heart attacks and strokes,” said Professor Schwaeble. “This new potential therapy was also shown in animals to significantly improve outcomes of transplant surgery and may be applicable to any surgical procedure where tissue viability is at risk due to temporary interruption of blood flow.
“The main focus of our work was to identify a key molecular mechanism responsible for the overshooting inflammatory response that can cause substantial destruction to tissues and organs following their temporary loss of blood supply, a pathophysiological phenomenon called ischaemia/reperfusion injury,” added Professor Schwaeble. “Limiting this inflammatory response in oxygen-deprived tissues could dramatically improve outcomes and survival in patients suffering heart attacks or strokes.”
For more than seven years, the University of Leicester team has been working closely with a commercial partner, Omeros Corporation in Seattle (USA), to develop therapeutic antibodies for research and clinical applications.
Omeros holds exclusive worldwide intellectual property rights to the MASP-2 protein, all therapeutic antibodies targeting MASP-2 and all methods for treating complement-mediated disorders by inhibiting MASP-2. The company has already begun manufacturing scale-up of an antibody for use in human clinical trials.
Professor Schwaeble’s team and Omeros are also working with Professor Nilesh Samani, the British Heart Foundation Professor of Cardiology and Head of the Department of Cardiovascular Sciences at the University of Leicester.
It is anticipated that the first clinical trials evaluating Omeros’ human antibody in myocardial infarction patients will be conducted in the Leicester Biomedical Research Unit , at Glenfield Hospital, Leicester.
Reference
1. Schwaeble QJ, et al. Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proceedings of the National Academy of Science (PNAS). Published online 18 April 2011