Microvisk secures £6 million to launch world’s first diagnostic SmartStrip
4 Feb 2011
UK company Microvisk Technologies, developer of a handheld system to monitor the blood clotting status of patients taking the drug Warfarin, has raised £6 million through a rights issue to existing investors in an oversubscribed round.
This is the third successful funding round for Microvisk, which has secured £10.5m in the past 12 months — the largest amount raised by a UK life science company.
The round included investment from Porton Capital, Oxford Technology Management, New Hill (Boston, USA), Midven, the Rainbow Seed Fund, Finance Wales and private investors. Previous rounds in 2010 saw Microvisk attract £4.5m from new and existing investors.
A spin-out company from the Science and Technology Facilities Council, Microvisk has developed the world’s first medical diagnostic strip based on a Micro-Electro-Mechanical System (MEMS) that was originally created as a movement system for nano-robots. MEMS technology is used in the computer projector, iPhone and Nintendo Wii as well as in car engines.
Microvisk’s ‘SmartStrip’ is a disposable strip that uses embedded sensors to work out the clotting speed of blood from a finger prick sample and the results are displayed on a handheld reader.
Microvisk is gearing up to launch its Smartstrip device onto the UK market this autumn. The company is using the new capital to continue clinical trials in the UK and expand its research and manufacturing facilities as well as recruiting additional scientists and manufacturing staff. Microvisk is also preparing to commence trials in the USA and Germany within the next six months, with product launches in both countries anticipated to follow in 2012.
Seven million people in the western world use Warfarin and the Food and Drug Administration (FDA) estimates that over one million new patients start taking the drug every year. Patients must have regular blood tests at their doctor’s surgery or hospital clinic to ensure they receive the correct dose. Warfarin is affected by food and exercise and if the dose is too low there is a risk of blood clots forming which can result in a stroke or heart attack, while too high a dose can lead to a life threatening bleed.
The Microvisk SmartStrip enables patients to test their blood clotting capacity at home, in the same way as diabetics test for glucose. As such, it reflects the trend towards healthcare monitoring in the home. In the past two years, the US and German healthcare systems have introduced payments to all at-risk Warfarin users to do weekly home blood tests, rather than having to go to the doctor or hospital clinic.
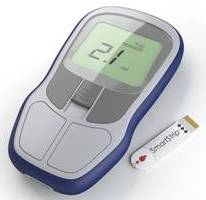
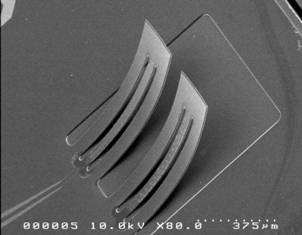
The Microvisk SmartStrip diagnostic system and
a close up of the cantilevers
To date, only three companies have developed a test system for blood coagulation that can be used in a doctor’s surgery and although certified for home use, market research shows that doctors feel that they are insufficiently robust and too complex for home use.
The Microvisk SmartStrip is unique in the blood clotting diagnostic world as a solid state system that is robust and simple to use at home. It also requires far less blood than other systems, which means less pain for the user. The coagulation status (clotting speed) of the patient is measured by tiny multi-layered paddles on the surface of the strip and a memory chip ensures the device is calibrated to provide the highest levels of accuracy.
John Curtis, chief executive officer of Microvisk, said: “We are delighted to have raised the funding needed to gear up for the manufacture and launch of our SmartStrip product in the UK this autumn. This funding round represents a real milestone for Microvisk as we also start to prepare for expansion into the German and US markets over the following year.”
Terry Swainbank, investment director at Synergis Technologies, part of the Porton Capital Group, said: “I congratulate the Microvisk team on their remarkable progress with the development of SmartStrip. We are pleased to support the company, which looks set to capture a significant share of the doctor’s office and home tests market for Warfarin patients.”
Dr Matthew Frohn, director of Oxford Technology Management, said: “Microvisk’s significant achievement has been to use a proven sensor technology in a medical diagnostic device and in so doing create a small, simple, handheld device that is perfect for home use. SmartStrip could revolutionise the way that blood testing is conducted in future.”
Dr John Mihell, investment executive at Finance Wales, said: “Microvisk is set to target a growing global market with its innovative SmartStrip product and Finance Wales is pleased to follow its investment in this round. We’re pleased to back the management team as they prepare to launch SmartStrip in the UK.”
Mark White of the Rainbow Seed Fund said: “The Rainbow Seed Fund was the very first external investor in Microvisk and we have continued to support the company over the past seven years. We are extremely encouraged by the technical and commercial progress that has been made and delighted that Microvisk now has an investor base strong enough to support it through to launch of SmartStrip. This oversubscribed round is vindication of our belief in the company’s initial promise and in the management and we look forward to a successful launch.”