ChromoTek launches RFP-Trap and GFP-Booster bioimaging reagents
2 April 2010
German company ChromoTek has launched two new bioimaging reagents, RFP-Trap and GFP-Booster.
RFP-Trap is used to identify and pull down interaction partners of proteins tagged with RFP (red fluorescent protein) and GFP-Booster restores or increases the GFP (green fluorescent protein) signal in super-resolution microscopy.
RFP-Trap is a versatile tool for biochemical and cell biological analysis of RFP-tagged fusion proteins in pull-down assays (a frequently used in vitro method used to determine physical interaction between two or more proteins).
Generally, pull-down experiments have been performed with antibodies, but as they are bulky molecules they tend to disintegrate during the purification process, making it difficult to further analyze the proteins of interest by mass spectrometry or the Western blot method.
ChromoTek has found a way to circumvent this problem by using small recombinant binding molecules, so called nanotraps instead of antibodies. Nanotraps bind their targets with similar efficiency but are only a tenth of the size of conventional antibodies and much more stable, thus allowing to effectively reduce background in pull down experiments.
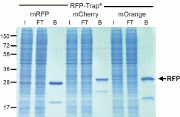
Figure 1.
The RFP-Trap pulls down all major
variants
of RFP (mCherry, mOrange) from cell
extracts
with minimal background
GFP is a powerful tool to study protein localization and dynamics in living cells. However, the photo stability and the quantum efficiency of GFP are not sufficient for super-resolution microscopy (e.g. 3D-SIM or STED) of fixed samples from cells expressing GFP fusion proteins to visualize specific structures. Furthermore, many currently used procedures for FISH or BrdU detection lead to disruption of the GFP signal. ChromoTek now has developed a GFP booster consisting of a chemical fluorescent dye that is covalently attached to a GFP Nanotrap thus providing high fluorescence intensities and signal-to-noise ratios.
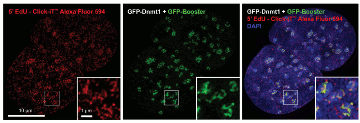
Figure 2. Enhancement of GFP signal with
GFP-Booster after EdU-Click-iT treatment. EdU-Click-iT™ treament
leads to disruption of GFP signal. GFP-Booster labels GFP fusion
proteins and thus reactivates and boosts the fluorescence.
Fluorescent proteins are indispensible markers that have enabled scientists to visualize molecular interactions within cells. The two most popular markers, GFP and RFP, are used in thousands of labs worldwide as tags for cellular proteins. GFP has proved to be so important for modern biomedical research that it earned the 2008 Nobel prize for its discoverers Osamu Shimomura, Martin Chalfie and Roger Y Tsien.