20-year breakthrough in HIV research
5 February 2010
Researchers at Imperial College and Harvard University have made a breakthrough in HIV research that had eluded scientists for over 20 years, potentially leading to better treatments for HIV. The study is published the journal Nature [1].
The researchers have grown a crystal that reveals the structure of an enzyme called integrase, which is found in retroviruses like HIV. When HIV infects someone, it uses integrase to paste a copy of its genetic information into their DNA.
Prior to the new study, which was funded by the Medical Research Council and the US National Institutes of Health, many researchers had tried and failed to work out the three-dimensional structure of integrase bound to viral DNA. New antiretroviral drugs for HIV work by blocking integrase, but scientists did not understand exactly how these drugs were working or how to improve them.
Researchers can only determine the structure of this kind of molecular machinery by obtaining high quality crystals. For the new study, researchers grew a crystal using a version of integrase borrowed from a little-known retrovirus called Prototype Foamy Virus (PFV). Based on their knowledge of PFV integrase and its function, they were confident that it was very similar to its HIV counterpart.
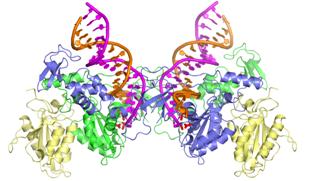
Drawing showing the structure of integrase
bound to viral DNA.
Credit: Imperial College London
Over four years, the researchers carried out over 40,000 trials, out of which they were able to grow just seven kinds of crystal. Only one of these was of sufficient quality to allow determination of the three-dimensional structure.
Dr Peter Cherepanov, the lead author of the study from the Department of Medicine at Imperial College London, said: “It is a truly amazing story. When we started out, we knew that the project was very difficult, and that many tricks had already been tried and given up by others long ago. Therefore, we went back to square one and started by looking for a better model of HIV integrase, which could be more amenable for crystallization. Despite initially painstakingly slow progress and very many failed attempts, we did not give up and our effort was finally rewarded.”
After growing the crystals in the lab, the researchers used the giant synchrotron machine at the Diamond Light Source in South Oxfordshire to collect X-ray diffraction data from these crystals, which enabled them to determine the long-sought structure. The researchers then soaked the crystals in solutions of the integrase inhibiting drugs Raltegravir (also known as Isentress) and Elvitegravir and observed for the first time how these antiretroviral drugs bind to and inactivate integrase.
The new study shows that retroviral integrase has quite a different structure to that which had been predicted based on earlier research. Availability of the integrase structure means that researchers can begin to fully understand how existing drugs that inhibit integrase are working, how they might be improved, and how to stop HIV developing resistance to them.
Reference
1. Stephen Hare, Saumya Shree Gupta, Eugene Valkov, Alan Engelman, Peter Cherepanov. Retroviral intasome assembly and inhibition of DNA strand transfer” Nature, January 2010. doi:10.1038/nature08784