Patient's own stem cells used to grow facial bone
29 October 2009
In a first-of-its kind procedure, stem cells taken from the fat tissue of a 14-year-old boy were combined with growth protein and donor tissue to grow viable cheek bones in the boy.
The new procedure dramatically improves the options surgeons have for repairing bone deficiencies caused by traumatic injuries — such as those from car accidents or soldiers wounded in battle — or by disease and genetic conditions, according to Jesse Taylor, M.D., a surgeon and researcher in the Division of Craniofacial and Pediatric Plastic Surgery at Cincinnati Children's Hospital Medical Center.
An estimated 7 million people in the United States have defects in bone continuity so severe that repair is difficult.
"We think this will benefit millions of people who, through traumatic injury or disease, have significant bone defects," Dr. Taylor explained. "The current methods we have — like borrowing bone from another part of the body, or implanting cadaver bone or something artificial — are reasonable alternatives, but far less than perfect."
Because the body rejects or absorbs implanted donor material, many reconstructive surgeries can have high failure rates. In procedures where bone is borrowed from one part of the body to replace another, the corrective surgery itself can be disfiguring to the person doctors are trying to help.
The new procedure avoids these problems because it uses the patient's own cells, Dr. Taylor explained. His team developed the procedure based in part on scientific research conducted in pigs at Cincinnati Children's. The operation is the first to blend and refine several techniques used or under study in surgical practice for repairing bone deficiencies.
The teenage recipient of the surgery, performed on May 28, has a rare genetic condition known as Treacher Collins syndrome, which includes underdeveloped or missing cheek bones. In this case the teenage patient, Brad Guilkey of Cincinnati, did not have developed zygomatic bones on either side of his face. The zygomatic bones form the prominence of the cheek and part of the outer rim of the eye socket.
The missing bones affected the active teenager's appearance, but more importantly put his eyes at increased risk of injury, Dr. Taylor said. The bones are supposed to surround most of the lower and side areas of the eye sockets, with a portion protruding toward the ear at the cranial base.
"This bone is critical structurally and acts as a shock absorber for the face, protecting the eyes and other critical structures in the event of facial impact," explained Dr Taylor. "This young man is extremely active, he loves to play basketball and baseball, and growing new bone in this area of his craniofacial structure is critically important for him."
Dr Taylor said the procedure has been successful and, more than four months after the surgery, computer tomography (CT) scans show the teenager's cheek bones have filled in normally with viable bone. The new bone structure enhances his appearance and improves protection for his eyes. Additional touchup surgery to the teenager's eye lids is under consideration to address a slight downward slant, also characteristic of Treacher Collins syndrome.
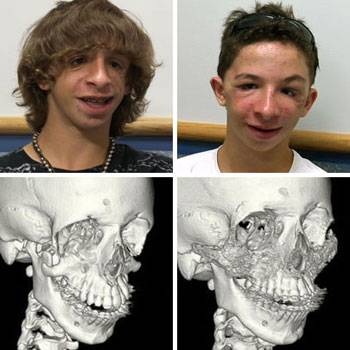
The photos at top left and right show 14-year-old Brad
Guilkey a week before his surgery and a few months post surgery.
Side-by-side images of CT scans taken before and after surgery reveal a
noted absence of zygomatic (cheek) bone structure on Brad's face (bottom
left); and the presence of healthy, dense bone structure a few months
following the May 28 procedure (bottom right).
Photo credit: Cincinnati Children's Hospital
During the day-long operation, surgeons used a section of donor bone to craft what essentially were mineral-based scaffolding implants (known as allografts), which also served as a growth guide for the new bone. Surgeons drilled holes in the allografts, which then were filled with mesenchymal stem cells taken from the patient's abdominal fat. Also injected into the allografts was a growth protein called bone morphogenic protein-2 (BMP-2) that instructs the stem cells to become bone cells called osteoblasts.
One of nature's roles for mesenchymal stem cells is to become cell types for a variety of different tissues in the body — including connective tissue and bone — giving the body a ready reserve of replacement cells as older cells die. In the surgery, and in the earlier lab experiments involving pigs, the doctors used BMP-2 to jumpstart nature's normal process of transforming these malleable stem cells.
"We only need to use a fairly small amount of bone morphogenic protein to serve as a cue to tell the mesenchymal stem cells that they're going to become bone," explained Donna Jones, Ph.D., a researcher at Cincinnati Children's and part of the scientific team that conducted experiments leading to the procedure.
"The actual molecular mechanisms BMP-2 uses to do this are not well understood, but once we use BMP-2 to start the process, the body's own biological processes take over and it produces its own BMP-2 to continue the transformation."
Particularly critical to that process is wrapping the donor allograft bone in a thin membrane of tissue that coats bone surfaces called periosteum. The periosteum used in this surgery was taken from the patient's thigh. Periosteum is important to the body's normal production of BMP-2, and just as vital to providing a blood supply to nourish new bone formation.
Drs Taylor, Jones and their fellow researchers are conducting ongoing studies into growing mandible bones in pigs. In a research paper being prepared for peer-review journal publication, they explain the use of the procedure to grow viable, dense bone in the animals and the duplication of results numerous times. The researchers worked with pigs because the porcine immune system is very similar to that of humans, making the animals a good model for simulating engineered bone growth in people.
Peer-review presentations of results from aspects of the study results have occurred at national re-constructive surgery conferences - including the American Association of Plastic Surgeons and the Plastic Surgery Research Council — and received with great enthusiasm, said Christopher Runyan, M.D., Ph.D., a member of the research team at Cincinnati Children's.
The team also plans additional research projects to test the procedure's ability to engineer bones of different lengths and sizes. Drs. Taylor and Jones said the technology may have the potential to grow almost any bone in the human body. As for Brad, now 15, and his mother, Christine, they're just happy Brad can play sports and participate in other activities without having to worry about a lack of facial bone making him more susceptible to serious eye injury.
"Until we had the CT scans before surgery, we had no idea that Brad was missing the bones that protect his eyes, and that's very dangerous," said Christine. "I was nervous about the procedure, but we're glad we did it and amazed with the results. The people at Cincinnati Children's do a great job of explaining things to you and we have a lot of trust in the doctors and staff."