Michelson Diagnostics issues preliminary specification for OCT probe
31 January 2009
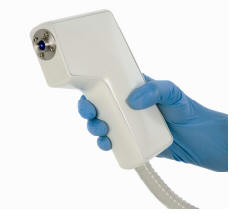 Michelson
Diagnostics has issued a preliminary specification for its clinical
Optical Coherence Tomography (OCT) probe and processing system, which
the company says is planned for launch in late spring 2009. The new OCT
system has been given the name ‘Vivosight’.
Michelson
Diagnostics has issued a preliminary specification for its clinical
Optical Coherence Tomography (OCT) probe and processing system, which
the company says is planned for launch in late spring 2009. The new OCT
system has been given the name ‘Vivosight’.
Two variants of the new Vivosight probe are specified, one is a version with X-Y scanning, to enable 3D mapping of flat tissue such as skin, and the other is a version equipped with a rigid endoscope, suitable for internal applications. The unique multi-beam design provides double the image resolution (ie better than 10 μm) available from conventional single-beam designs, resulting in crisper, clearer OCT images, showing more clinical detail.
“We will be applying for a CE-mark for the product, which will enable
sales for clinical use in Europe, in the spring 2009 time-frame.” said
Jon Holmes, Chief Executive, “This will be followed by a 510(k) for the
equivalent in the US market.”
Bookmark this page