Intel receives FDA clearance for personal health system
14 July 2008
Intel Corporation has received 510(k) market clearance from the US Food and Drug Administration (FDA) for its personal health system, the Intel Health Guide, a care management tool for healthcare professionals who manage patients with chronic conditions.
The Health Guide enables caregivers to provide their patients with more personalised care at home, while also engaging and empowering patients to take a more active and positive role in their own care.
The Health Guide is a comprehensive solution that combines an in-home patient device (see photo below), as well as an online interface allowing clinicians to monitor patients and remotely manage care. The system offers interactive tools for personalised care management and integrates vital sign collection, patient reminders, multimedia educational content and feedback and communications tools such as videoconferencing and email.
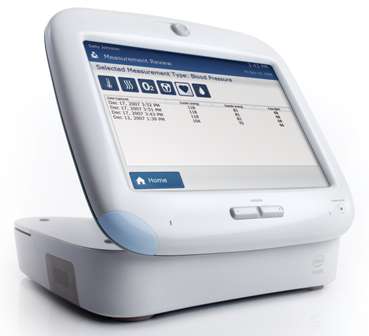
The in-home patient device of the Intel Health Guide system
The Health Guide can connect to specific models of wired and wireless medical devices, including blood pressure monitors, glucose meters, pulse oximeters, peak flow meters and weight scales.
The Health Guide stores and displays the collected information on a touch screen and sends to a secure host server, where healthcare professionals can review the information. Patients using the Health Guide can monitor their health status, communicate with care teams and learn about their medical conditions. The patient must have broadband to their home to access the online services.
The Health Guide PHS6000 patient device features a 10.4" colour touch screen, integrated VGA video camera, microphone and speaker, four USB 2.0 ports, Bluetooth wireless, RJ-45 network connector, 40GB password protected disk drive, 128-bit encryption and secure socket layer. It weighs about 3.8kg and has the footprint of a small laptop computer (280x90x270mm).
“This is an important product that will improve the state and cost of healthcare around the world,” said Louis Burns, vice president and general manager of Intel’s Digital Health Group.
“It results from years of research to understand the needs of the aging population and how technology can support them in their daily lives. With more people living with chronic diseases, we believe care can be increasingly moved outside of the hospital to the home. Through our research, we’ve learned that a home-based model of care becomes more than just delivering care to patients at home — it is about creating connections to family, friends, caregivers, and the care team.”
“We believe the Intel Health Guide represents a new category of personal health systems that goes beyond the simple remote patient monitoring devices available today,” Burns said. “We envision a wide range of usage models, not only chronic conditions such as CHF and diabetes, but also programs for health and wellness management at home.”
Intel has completed pilot studies of the system in the United States and UK. Later this quarter, Intel will conduct additional pilots with healthcare organisations to understand how the guide integrates with different care management models in the home. Intel says it expects the guide to be commercially available from healthcare providers in the US and UK in late 2008 or early 2009. The system is pending CE approval for sale in the EU.