| Diagnostic imaging, nanotechnology | |||
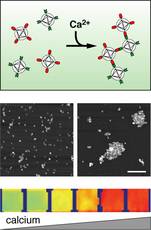
Magnetic nanoparticles enable magnetic resonance imaging of neuron activity in brain7 November 2006 Cambridge, USA. New chemical sensors that indicate the firing of neurons in the brain and show up strongly in magnetic resonance imaging will open the way for new research into the way the brain works. Neuroscientists eagerly anticipate the day when they can use non-invasive brain imaging to see precisely what the 10 billion neurons are doing throughout a person’s brain. But a trio of limitations facing current functional magnetic resonance imaging (fMRI) technology stands in the way of that goal: time, space, and specificity. At the McGovern Institute for Brain Research at the Massachusetts Institute of Technology (MIT), Alan Jasanoff is developing new chemical sensors that are detectable by MRI machines and will overcome these limitations. The first of these tools, a nano-sized calcium contrast agent, is reported in the October edition of the Proceedings of the National Academy of Sciences.
“Using conventional fMRI to study the brain is like trying to understand how a computer works by feeling which parts of it are hot because of energy dissipation in different components of the machine,” explained Jasanoff. “But chemical sensors for MRI could show what each individual element in each integrated circuit is doing and how it performs the computations and processes information.” The analogy is apt, because fMRI indirectly measures neural activity by detecting changes in blood flow to brain regions with increased energy requirements. However, these homodynamic changes occur several seconds after the neurons actually fired, too slow to study precise neural activity. The spacing of the capillaries limits the spatial resolution of the technique to volumes containing at least 1,000 neurons, too coarse for discrimination of highly specialized functional areas within a brain region. Calcium, however, provides a direct measure of neural activity because calcium almost instantly flows into neurons when they fire, and the faster the rate of firing, the higher the calcium level. Thus, tracking calcium levels in the brain actually tracks information flow through the brain’s circuits. “The changes are pretty dramatic,” Jasanoff said. “Calcium concentrations can vary by more than an order of magnitude, and the fluctuations are relatively long-lasting.” Jasanoff drew his inspiration for this contrast agent from optical microscopy, which uses light to study cellular properties and has long targeted calcium as a way to image neural signals. But optical microscopy can only penetrate about 2 millimeters and cannot image the deeper brain tissues, while MRI could detect contrast agents throughout the whole brain. To be visible to MRI, which detects changes in magnetic properties, a contrast agent must include a magnetically-active “paramagnetic” component. Previous approaches to designing MRI calcium sensors used only one or two metal atoms, to create a relatively weak magnetic effect that required high concentrations of the agent for detection. Such high concentrations of a calcium-sensitive agent could potentially swallow up enough calcium to interfere with neural circuitry in vivo. To overcome this limitation, Jasanoff designed the sensor to incorporate so-called “superparamagnetic nanoparticles” — extra strength molecular-sized magnets that had previously been designed for ultrasensitive tumour imaging. Because the new calcium sensor based on these particles produces large MRI contrast changes, it may be used at low concentrations that will not perturb organisms. Jasanoff’s sensor is actually made from two similar types of superparamagnetic nanoparticles. One is attached to a short corkscrew-shaped protein segment called M13, and the other is attached to another protein called calmodulin, that binds to M13 in the presence of calcium. When calcium levels rise, the two types of particle stick to each like Velcro-coated balls. They form aggregates that in turn affect MRI contrast. A characteristic of sensors that work this way is called “T2 relaxivity.” This property makes the sensors suitable for use in powerful MRI scanners capable of producing very high-resolution images. Also important to the new calcium sensor’s function is the fact that its aggregation and readout are reversible. This property allows it to indicate the temporal dynamics of calcium-related neural activity, such as the sequence in which populations of cells become active, or the synchronization of neurons during certain behaviours. Jasanoff is currently working on non-invasive methods to deliver the calcium sensor to brain cells in vivo, focusing on small animals like flies and rats. Graduate student Tatjana Atanasijevic, who is the lead author on the PNAS paper, is optimizing properties of the sensor for applications in animals. Meanwhile, others in Jasanoff’s group are changing the makeup of the nanoparticle so that it can target specific genetic characteristics or different populations of neurons, such as inhibitory or excitatory neurons or those that produce specific neurotransmitters. “These will be tools for making the shift from imaging gross functional properties of the brain through its haemodynamic changes to a fine tuned analysis based on information flow involving cells and circuits,” Jasanoff said. “There are many potential applications for studying learning, memory, and behaviour, and we need the new tools to get to the applications.” In addition to his appointment as an associate member of the McGovern Institute, Jasanoff is Assistant Professor in the Departments of Nuclear Science & Engineering, Brain & Cognitive Sciences, and Biological Engineering Division. This research is supported by grants from the Raymond & Beverley Sackler Foundation and the NIH/NIBIB, and a McKnight Foundation Technological Innovations in Neuroscience award.
|