| Diagnostic imaging | |||
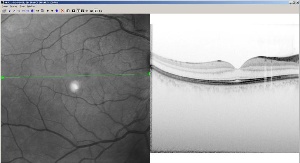
Heidelberg's high-speed retinal imaging device receives US clearance6 November 2006 Heidelberg, Germany. Heidelberg Engineering GmbH has been granted US clearance for its groundbreaking Spectralis HRA+OCT retinal imaging device. The company claims it is the first commercial retinal imager to combine a spectral domain optical coherence tomograph (OCT) with laser angiography. This ground breaking development provides clinicians with unprecedented details of the structure of the retina. This new product detects previously unrecognized structures, combining high resolution cross-sectional images of the retina with any of four imaging modalities: autofluorescence, infrared, fluorescein angiography, or ICG angiography.
The new device scans the retina 100 times faster than older existing technology known as time domain OCT. The Spectralis HRA+OCT is a spectral domain system, sometimes called fourier domain, which scans the retina at 40,000 scans per second, creating highly detailed images of the structure of the retina. Because the OCT and Heidelberg Retina Angiograph (HRA) images are captured simultaneously, the clinician can be assured of the exact location of the area of interest and can correlate the outer visible retina structure with the internal structure. “This new technology represents a dramatic leap in our ability to image complex macular disease,” said Scott Cousins, MD, director, Duke Center for Macular Diseases, at Duke University. “The combination of these two cutting edge technologies will amplify our understanding of retinal structure and provide us with new insights into the biological processes of the retina.” The new product is built on the company’s successful Heidelberg Retina Angiograph (HRA) platform, the first commercial angiography system to use lasers in combination with marker dyes such as sodium fluorescein and indocyanine green (ICG). Using the HRA instead of white light photography has allowed clinicians to capture detailed images of the blood vessel structure within the retina, a key diagnostic indicator for such common eye disease as age-related macular degeneration and diabetic maculopathy. Another advantage of using lasers is the fast frame rate which enables movies of the blood flow, adding a new diagnostic dimension over traditional photography. Of recent interest is the HRA’s capability to cause certain retinal components to fluoresce in a process known as autofluorescence. Geographic atrophy of age-related macular degeneration is being followed using autofluorescence as a potential early indicator of disease progression in the recently announced AREDS 2 clinical trial. The new product will be presented at the upcoming American Academy of Ophthalmology meeting in November. The company expects to begin shipping the product in mid-2007.
|