| Diagnostic imaging | |||||||
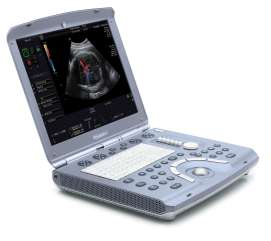
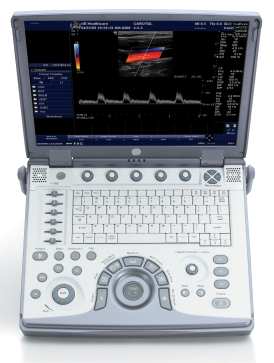
GE introduces new series of laptop-size ultrasound systems26 May 2006 Waukesha, Wis., USA & Seoul, Korea. GE Healthcare is introducing four new clinically specialized ultrasound systems to address the growing demand for diagnostic imaging at the point of care. GE's Compact Series places the power and imaging capabilities of a high-performance, 400-pound system into a laptop-size design. The new ultrasound systems are being launched at the World Federation of Ultrasound in Medicine and Biology congress in Seoul, Korea (May 28 – June 1, 2006). The Compact Series builds upon GE's successful introduction in 2005 of Vivid i, a high-performance cardiovascular ultrasound system in a compact design. Blending image quality and portability with clinical applications, reporting tools and a user interface designed specifically for cardiac imaging, Vivid i has been rapidly adopted by the medical community, and today is transforming the way heart disease is detected, diagnosed and managed at more than 1,000 sites around the world.
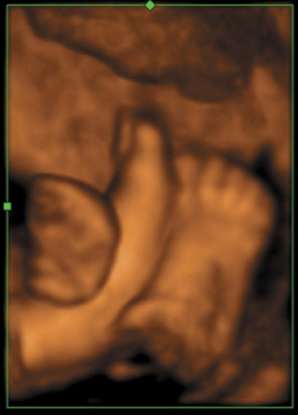
GE's Compact Series has now been expanded to include the new Voluson i and LOGIQ i systems — each "i" product is designed to bring specialized, console-quality imaging performance and portability to traditional applications. Voluson i is designed for Obstetrics & Gynecology applications, while the LOGIQ i will serve the general imaging needs of radiology. GE's "e" products focus on expanding ultrasound's reach to new clinical areas. The new LOGIQ e was designed with the speed requirements and imaging applications to support real-time clinical decisions in emergency and surgical settings. GE's new Vivid e provides a dedicated cardiac ultrasound imaging solution for the Physician Office in a practical, easy to use design. According to Omar Ishrak, president and CEO of GE Healthcare's Clinical Systems division, miniaturization was the first step in bringing basic ultrasound to the point-of-care. "Until now, the broad adoption of compact ultrasound has been hindered by image quality limitations and the industry's 'one size fits all' approach to compact system design," said Ishrak. "By working with physicians from a wide-range of medical specialties, we've learned that image quality, portability and clinical specialization are all essential to expanding ultrasound's role in healthcare. We've developed our new Compact Series to address these needs and bring the benefits of ultrasound to virtually all clinicians and patients — creating a pathway for ultrasound to become as ubiquitous in patient care as the stethoscope is today." According to Ishrak, who has more than 20 years in ultrasound technology development and has served as the leader of GE's ultrasound division since 1995, "GE's clinical partnerships, technology leadership and continuous investment in ultrasound allows us to invite healthcare's top physicians 'to the drawing board' to re-imagine ultrasound. Together, we're developing innovative ultrasound systems to help address some of today's most pressing healthcare issues such as improving access to quality care in rural communities and developing regions of the world, and in developed regions, shifting to an 'early health' model where technologies such as ultrasound can be used to help detect diseases earlier when they can be more effectively treated." GE's Voluson i, LOGIQ e and Vivid e systems are FDA cleared and will become commercially available in the U.S. in June 2006. LOGIQ i is also FDA cleared with plans for commercial introduction in September 2006. Vivid i received FDA clearance in 2004 and was commercially available in early 2005.
|