| Nanotechnology | |
Carbon nanotubes wrapped in DNA act as optical sensors in cells3 February 2006
When the DNA is exposed to ions of certain atoms — such as calcium, mercury and sodium — the negative charges become neutralized and the DNA changes shape in a similar manner to its natural shape-shift from the B form to Z form. This reduces the surface area covered by the DNA, perturbing the electronic structure and shifting the nanotube’s natural, near infrared fluorescence to a lower energy. “The change in emission energy indicates how many ions bind to the DNA,” said graduate student Daniel Heller, lead author of the Science paper. “Removing the ions will return the emission energy to its initial value and flip the DNA back to the starting form, making the process reversible and reusable.” The researchers demonstrated the viability of their measurement technique by detecting low concentrations of mercury ions in whole blood, opaque solutions, and living mammalian cells and tissues — examples where optical sensing is usually poor or ineffective. Because the signal is in the near infrared, a property unique to only a handful of materials, it is not obscured by the natural fluorescence of polymers and living tissues. Source: University of Illinois at Urbana-Champaign Link University of Illinois Department of Biochemical and Molecular Engineering www.scs.uiuc.edu/chem_eng/
|
 “This
is the first nanotube-based sensor that can detect analytes at the
subcellular level,” said Michael Strano, a Professor of Chemical and
Biomolecular Engineering at Illinois and corresponding author of a paper in
the Jan. 27 issue of the journal Science. “We also show for the first
time that a subtle rearrangement of an adsorbed biomolecule can be directly
detected by a carbon nanotube.”
“This
is the first nanotube-based sensor that can detect analytes at the
subcellular level,” said Michael Strano, a Professor of Chemical and
Biomolecular Engineering at Illinois and corresponding author of a paper in
the Jan. 27 issue of the journal Science. “We also show for the first
time that a subtle rearrangement of an adsorbed biomolecule can be directly
detected by a carbon nanotube.”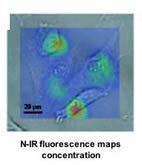 At
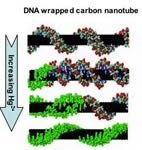
the heart of the new detection system is a change in the structure of
DNA from the normal, right-handed “B” form to the alternate, left-handed “Z”
form. This occurs in the presence of certain chemicals and changes the
wavelength of light emitted by the DNA, which can be detected and used to
produce images. To make their sensors, the researchers begin by wrapping a
piece of double-stranded DNA around the surface of a single-walled carbon
nanotube, in much the same fashion as a telephone cord wraps around a
pencil. The DNA starts out wrapping around the nanotube with a certain shape
that is defined by the negative charges along its backbone.
At
the heart of the new detection system is a change in the structure of
DNA from the normal, right-handed “B” form to the alternate, left-handed “Z”
form. This occurs in the presence of certain chemicals and changes the
wavelength of light emitted by the DNA, which can be detected and used to
produce images. To make their sensors, the researchers begin by wrapping a
piece of double-stranded DNA around the surface of a single-walled carbon
nanotube, in much the same fashion as a telephone cord wraps around a
pencil. The DNA starts out wrapping around the nanotube with a certain shape
that is defined by the negative charges along its backbone.