Case study
Barcodes and handheld computers ensure quality assurance for MED-EL ear implant production
4 August 2009
Summary
Tyrol-based manufacturer MED-EL and its subsidiary Vibrant-Medel develop, produce, and market the high-technology ear implant hearing devices. Strict US and European regulations require seamless product documentation and traceability of every individual part throughout the life cycle of the product. This process has been streamlined by a system integrated by B&M that uses Datakey ERP middleware and Honeywell Dolphin wireless mobile computers with data capture by 2-D barcodes. The system reduces manual errors, eliminates paper documentation, and increases the security of all logistics processes.
Company overview
In 1975, two Austrian scientists and the founders of MED-EL, Prof. Erwin Hochmair and Dr Ingeborg Hochmair, developed the first hybrid multi-channel ear implant. Since then, the university spin-off has developed into a globally active enterprise that offers a broad range of ear implants for various types of hearing loss. The MED-EL product range comprises the cochlear implant system Maestro, the EAS hearing system for combined electric acoustic stimulation, and the Vibrant Soundbridge, the world's first approved middle-ear implant.
The challenge of FDA-compliant quality assurance
"The Medical Devices Act requires us to document every single implantable part from production to the entire life cycle of the implant as well as the patient’s life,” explains Dr Walter Fimml, IT Manager at MED-EL. “As more than 25 digits needed to be entered manually in some instances (5 or 6-digit S/N), errors were unfortunately introduced during the input of serial numbers.
"According to the Medical Devices Act, we must be certified according to ISO EN 13485, which is a lot of work. Currently, more than 20 people work in MED-EL's Quality Management (QM) department alone. In addition, there are QM officers in the individual departments. Almost ten percent of our staff members are responsible for quality management and quality assurance."
"Every year, FDA officers conduct audits that take several days,” explains Werner Goth, Warehouse Logistics Manager at MED-EL. “For us, this means that we have to prepare documentation and take numerous audit steps, all of which ultimately serve the security of the patients."
Cross-sectional drawing of the ear showing
the position of the ear implant.
Tracing goods logistics at MED-EL
An ear implant system consists of implantable and external parts. Implantable parts require sterile packaging, which is a challenging base for printing barcodes. The miniature component groups and parts must bear the serial number and be labelled in the appropriate local language to enable the patient to identify the package contents. The systems are shipped to hospitals and physicians in handy cases along with software and documentation. Logistics therefore play a key role in the distribution of these highly sensitive products.
The components are transferred from the incoming goods department to the main warehouse, where they are either directly selected for production or for external partners. The products are prepared for dispatch in the warehouse. "Unlike conventional warehouses, the requirements for our warehouse are very high," adds Goth.
"An advanced degree of responsibility and precision is vital for our shipping staff. Our warehouse team is made up of specialists who conduct customer-specific configuration, assembly, and electronic adjustment tasks for each order. In this context, they now benefit from the support of the B&M wireless data system."
The solution
MED-EL first contacted integrator B&M at a professional trade show. "We were looking for a wireless data system that would meet our advanced security requirements,” recalls Dr Fimml. “The B&M team understood our needs and was able to meet these to provide a practical, functional, and secure solution."
MED-EL already had an encrypted high security wireless local area network (WLAN) installed, and only needed additional access points for the warehouse area. The company chose a system using 2-D barcodes and Honeywell handheld computers.
B&M delivered Honeywell Dolphin 7850 wireless computers and connected them to the Navision Enterprise Resource Planning (ERP) system via DATAKEY middleware. From the DATAKEY control station, the warehouse manager can selectively issue picking orders, for example in order to release specific orders to particular individuals, depending on the qualification of the team members.
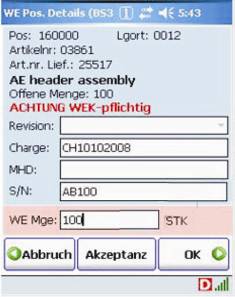
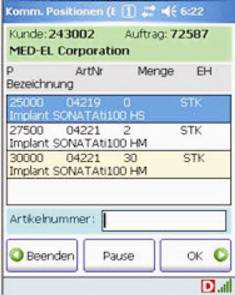
Screenshots of the ERP system on the Dolphin 7850
Apart from facilitating administration, this approach is more secure than the previous system which used printed order slips which virtually anybody could take. "We did not encounter any major problems during the project rollout and we were very satisfied with the project flow," explains Dr Fimml, IT Manager at MED-EL. "We have eliminated manual errors and the process has become faster and more secure."

Barcode scanning using the Dolphin 7850 mobile
computer
Serial number tracking
The mobile data collection begins with incoming goods. Most of the supplied parts arrive in cardboard boxes that are already marked with barcodes or are labelled at the company upon arrival. MED-EL uses code 39 for box labelling. Boxes that are not already labelled are equipped with barcode labels after registration. The label printing is triggered directly from the mobile computer.
One of the most outstanding features of the MED-EL warehouse is the great variety of products, most of which are stored in Rotomat systems. Currently more than 6,000 different products are kept in stock for the production of MED-EL ear implants. There is also a storage area for auxiliary supplies and one for packaging and marketing material.
When the implant leaves production, it already has a serial number and a documented manufacturing history. Before an ear implant customer set is assembled, the serial number is scanned. The scanning of the serial number plays a significant role in minimizing documentation errors.
The benefits
"The Honeywell mobile computers are now directly connected to Navision ERP system over the DATAKEY middleware, and all tasks can be performed on site using the mobile computers such as querying orders, scanning goods, correcting quantities, etc,” explains Alois Greiderer, ERP Manager at MED-EL. “This saves the team a lot of time and reduces input errors."
"The mobile data collection mainly serves the following objectives: the error rate during data collection should approach zero, part of the paper documentation will be eliminated, and the security of all logistics processes will be increased," adds Dr Fimml.
The future
Except for China and Turkey, no other country explicitly demands barcodes on medical devices — even in the USA, no standard is expected to be adopted before 2015. "Naturally, we want to have a system that is sustainable and internationally recognized,” says Dr. Fimml.
“Therefore, we currently use the DataMatrix code for all small parts. Should this code not be adopted as the standard in 2015, we will have to change it. Nevertheless, we will be able to draw on our long-standing experience and get started on the basis of an operable system."
"Due to the high quality requirements, every change to our system is very time-consuming,” adds Werner Goth. “For instance, changes to the sterile packaging of the implants are subject to elaborate approval processes. Therefore, we expect the full migration to paperless picking to take at least three years. Until then, to be on the safe side we will use both methods, ie wireless computers and order slips."
At any rate, the introduction of the B&M wireless system forms the basis for consistent, seamless data, batch, and serial-number input and paperless logistics handling. In keeping with MED-EL's traditional style, this will be followed by further steps on the road to perfection.
Bookmark this page