Lab-quality point-of-care test for hypothyroidism promises measurable health benefits
Undiagnosed thyroid disorders are a worldwide problem. Testing for hypothyroidism has required central laboratory facilities and follow-up procedures to get patients into and stay in treatment. Vivacta has now developed an immunoassay test that can be delivered at the point-of-care. The test kit consists of a portable bench-top reader and a credit-card-sized disposable cartridge to take and analyse a pin-prick of blood. Based on piezofilm technology it can analyse whole blood for TSH in just five minutes, allowing, for the first time, testing, reporting and treatment to be done in the same doctor's appointment.
1 December 2008
Thyroid disorders affect more people worldwide than diabetes, and even ‘mild’ cases can endanger health. Recent clinical studies reveal that mild hypothyroid conditions can significantly increase the risk of cardiovascular problems, including raising the chance of heart failure twofold [1].
However, although hypothyroidism is easy to treat using a drug with no side effects, more than 50% of people in developed regions such as the US and Europe remain undiagnosed. Furthermore nearly 50% of people diagnosed do not adequately control their condition and maintain normal range of key markers.
The problem with current testing procedures
Part of the problem is that the key marker for thyroid function, thyroid stimulating hormone (TSH), which requires high sensitivity, has to-date only been performed in central laboratories. TSH can distinguish even subclinical hypothyroid cases better than, for example, the ‘free’ thyroxine (FT4) test [2].
However, the need to collect a sample suitable for laboratory testing and send it away reduces the likelihood that a patient will be diagnosed and appropriately treated, especially in areas that lack fully-equipped laboratories. Patients referred to a third party laboratory by their doctor often 'no show'. Others may be put off testing because they are squeamish about providing a tube of venous blood.
As with all laboratory tests, patients often don’t follow-up their results, and even when they do receive their results, they often only receive a brief follow-up telephone call if their thyroid condition is marginally outside of the normal range, ie subclinical.
Requirements for PoC testing
A point-of-care (PoC) test for TSH and other conditions would benefit patients and clinicians. Test results can be delivered and discussed in detail while the patient is in the surgery, and where any necessary thyroxine medication dose adjustments can be made.
However, moving tests, such as TSH, from the lab to the point-of-care has been held back by the limitations of current PoC technologies. A common type of PoC test is the lateral flow test (based on an immunochromatographic strip), commonly used for detecting pregnancy, HIV, infectious diseases, respiratory diseases and drug abuse, among other applications.
Lateral flow tests, however, are normally qualitative and at best
semi-quantitative and lack the required sensitivity. This makes them
unsuitable for tests like TSH, which require sub picomolar sensitivity
and an approximately 10,000 to 1 dynamic range. Lateral Flow technology
also requires consideration of the IP freedom to operate.
Other technologies for immunochemistry PoC testing include variants on
lateral flow: fluorescent labelled systems, that provide a quantitative
measurement by effectively counting photons, are a common approach.
A disadvantage with such systems is the inherent need for complex optics, driving up costs and making them more difficult to operate. As most of these tests use optics to make a measurement, or require the user to interpret a colour change, which means they cannot be used with whole blood since it is coloured and semi-opaque.
As a result, these tests require a red cell separation step which not
only adds complexity to the disposable cartridge but also provides
another IP field to be circumnavigated. Furthermore, PoC tests should
ideally use finger-prick samples of capillary blood, which limits the
volume to 30 microlitres. Although this is more than adequate for a
glucose test, it is difficult to separate plasma from cells without
significant sample loss.
A PoC test suitable for demanding and high volume applications in
primary care, like TSH testing, needs to have a dynamic range of more
than 5,000 and a minimum detection limit of circa 10 pg/mL. It must
provide a result while the patient is in the doctor’s surgery (within a
maximum of 10 minutes) using a single drop of capillary blood from a
finger stick, ie 30 µl volume or less.
Finally, it must do these things while being of low enough cost to allow wide adoption, and simple enough to achieve US FDA CLIA-waived status. The latter means the test can be carried out by a non-expert user and needs to “employ methodologies that are so simple and accurate as to render the likelihood of erroneous results negligible; or pose no reasonable risk of harm to the patient if the test is performed incorrectly” [3].
With such exacting requirements, it is no wonder that current PoC tests have failed to meet them, until now. A new type of immunoassay test that uses piezofilm technology, is now poised to deliver TSH testing at the point of care.
The answer: piezofilm technology
The piezofilm technology can analyse whole blood in just five minutes and is simple enough to package into a small, self-contained cartridge. Furthermore, it has an analytical sensitivity of circa 10pg/mL and a dynamic range of 1,000 – 10,000.
The developer of the technology, Vivacta, intends to develop the demanding TSH test as a proof-of-concept of the technology’s broader capability.
TSH also makes an excellent test-bed for this technology because it is a standalone test. Later, the company aims to introduce additional supporting thyroid tests. It also intends to introduce other tests for use in endocrinology clinics and doctor’s surgeries, such as diabetes management and female and male hormone markers.
How it works
The Vivacta system consists of a bench-top, portable reader and a single use, disposable cartridge, which integrates with the whole blood collection device. As blood is drawn directly into a capillary channel in the cartridge from a finger stick (see figure 1) no venipuncture is required, minimising patient distress. An indicator on the cartridge shows when it is full.

Figure 1: Blood is drawn into the cartridge from capillary fill
On one side of the channel is a piezofilm. This is a poled polyvinylidene fluoride (PVDF) polymer film which generates an electric charge when a rapid thermal event is experienced, or if a mechanical strain is applied. This is the piezoelectric effect, first documented in 314 BC by the Greek Theophrast [4].
Bound to the piezofilm is an antibody or antigen specific to the target analyte. The capillary channel also has ‘reporter’ labels dried onto it, which have target analyte-specific antibodies adsorbed onto their surface. The blood resuspends these reporter labels, which bind to the target analyte and, subsequently bind to the antibodies mounted on the piezofilm. A 'sandwich' is formed consisting of the reporter label, the target analyte and the antibodies on the piezofilm (the ‘capture’ antibodies) (see figure 2 below).
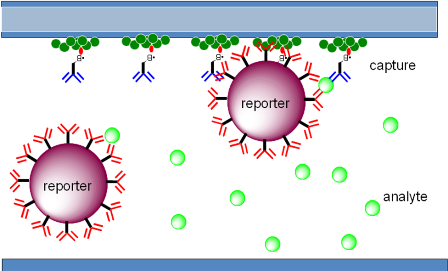
Figure 2. The capillary channel showing the analyte binding to the resuspended reporter labels and, subsequently, forming a sandwich with the capture antibodies bound to the piezofilm.
While the reporter label is binding to the piezofilm, a bright LED is flashed through it. Although the LED wavelength is minimally absorbed by the red cells, the reporter has been specifically chosen to absorb the light and convert it into heat. Reporter labels bound to the piezofilm dissipate this thermal pulse into it, causing an electric charge to be generated.
The voltage generated increases at a rate directly proportional to the rate of binding of the target analyte to the surface, and therefore its concentration in the blood sample (see figure 3 below). This means that the piezofilm system provides a quantitative, rather than a qualitative result, as with lateral flow tests.
Furthermore, because a linear rate of binding is measured, a result can be generated as soon as enough of the reporter label and analyte has bound to the surface. This can happen in as little as a one minute or five minutes when a low detection limit is required, in the case of TSH.
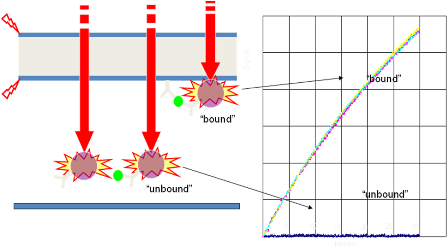
Figure 3. Bound reporter labels dissipate their heat into the piezofilm (flash) when illuminated (red arrows), generating an electric charge (red lightning symbols) and a linearly rising voltage.
Unbound reporter labels dissipate their heat into the blood sample without generating a voltage in the piezofilm, thereby allowing bound and unbound label to be distinguished in a whole blood sample without the need for a washing or separation step. This, along with the simple LED light source and voltage measurement device, allows the whole system to be contained in a small, easy-to-use reader and cartridge. Furthermore, the analytical sensitivity of the system is very high, circa 10 pg/ml (see figure 4).
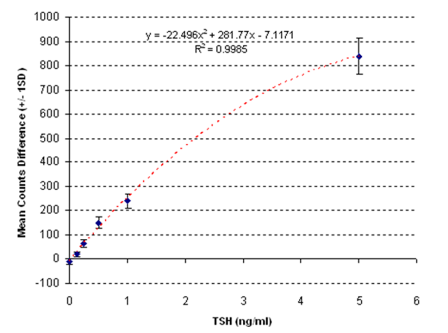
Figure 4: Typical calibration plot showing dose to signal response.
Applications
The piezofilm technology TSH test has several applications. First, it can be used in thyroid screening, as discussed. Overt hypothyroid conditions can be diagnosed and treated and subclinical thyroid conditions can be diagnosed, treated and regularly monitored all during a single doctor’s appointment.
Once a hypothyroid case has been diagnosed, patients are likely to be placed on Thyroxine medication, the dose being gradually being increased with regular TSH monitoring to ensure the patient's TSH is brought into the normal range without risk of overdose. This single 'test and treat' regime provides physicians with a much more efficient process than the earlier ‘test at one appointment, treat at another’ approach’.
Additional opportunities exist for a rapid TSH test, where hyperthyroidism can introduce complications for patients who are administered contrast agents prior to an imaging procedure. A rapid test that can be performed before an emergency angiogram or acute imaging procedure would eliminate rushing samples to central lab.
Conclusion
In conclusion, immunoassay tests requiring high sensitivity, such as the TSH test for thyroid function, have to date been solely provided by a central lab because of their exacting requirements.
Bringing the test to the point of care provides physicians with a more efficient and effective ‘test and treat’ regime, improving compliance by involving the patients in the result and eliminating no-shows. This is being enabled by the new PoC technology, which uses the piezoelectric effect to produce precise, quantitative diagnoses, while being simple and small enough to be used in a doctor’s office.
References
- Rodondi N, et al. Subclinical Thyroid Dysfunction, Cardiac Function and the Risk of Congestive Heart Failure: The Cardiovascular Health Study. ATA meeting 2007; Program number 92.
- Spencer CA, et al. Applications of a new chemiluminometric thyrotropin assay to subnormal measurement. Journal of Clinical Endocrinology & Metabolism, Vol 70, 453-460, 1990.
- http://www.fda.gov/cdrh/clia/cliawaived.html
- Caley ER and Richards JFC. Theophrastus on Stones. The
Ohio State University, Columbus, 1956.
Bookmark this page