Personalising healthcare with technology
Mark Brincat, Director of Product Strategy, Exco InTouch
2 May 2014
Adaptive solutions
The products and services that support patients in managing their health and diseases must be able to adapt in the real world to individual patient needs. As technology develops we should be seeing a continual evolution in dynamic and adaptive solutions that can support patients on their individual ‘patient journeys’. There are no hard and fast rules as to how adaptability is defined, but there are a number of levels that help understand the extent to which a product might respond to individual patient needs.
Perhaps the highest level to start with is personalisation. This is where a patient can put their own mark on a product and make it feel like something that is unique to them, perhaps including some elements of look and feel, or methods of interaction. Next, a patient could configure a product around their own specific requirements. The product might have been already configured with a patient’s clinical specifics by a healthcare professional, but patients could additionally configure to their own needs and priorities.
Once the patient is up and running, the product would be designed to respond to a series of patient inputs and events, some adhoc and some scheduled. It is here that products are really going to differentiate themselves. These pathways are numerous and complex and a product must be flexible enough to respond to different patient profiles, requirements and progressions. Multi-level solutions might include assessment, medications management, lifestyle management and informational content, all of which need to work and adapt in sync with each other.
For example, if assessment and medication tracking show a change in condition, then there is a likely need to reflect changes in lifestyle management and informational support. Add on top of this a patient’s state of behaviour acceptance and there is a variable set of parameters that a product needs to interpret and respond to correctly.
Looking at the technology involved, this would require a rules engine to manage a dynamic set of interactions. At its simplest level, a rules engine can be seen as software that uses rules that can be applied to data to produce outcomes. It is important that rules are only defined where events and outcomes are sufficiently understood.
In the future, expert systems will take findings from the system and dynamically build them back into the rules engine, so that a solution learns and improves interventions based on real world data. For now, anonymised patient data findings are used to refine or build new rules and interventions into the system.
Companies will need to develop skills around the analysis of ‘big data’, identifying signals and patterns. This will be an exciting period in advancing our understanding of patient populations and disease anthropology.
When patients stop taking medication or stop proactively managing their condition generally, their change of mind did not happen that morning, it started weeks or months ago with a series of smaller issues slowly stacking up. mHealth solutions need to focus on the multitude of issues and support patients with a range of interactions that work in sync with each other and respond to an individual patient’s real world experience (as seen in the Interventions Wheel in figure 1). As this level of mHealth product takes hold in the market, it will be remarkable how quickly we can advance disease management.
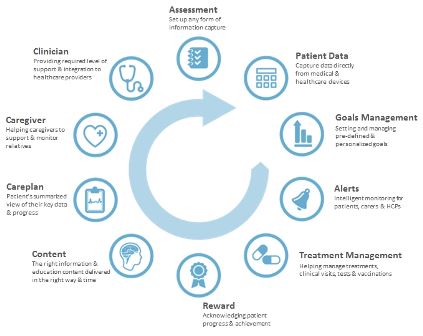
Figure 1. The interventions wheel
Changing behaviour
In order to achieve the desirable long-term outcomes for patients with chronic diseases we have to get to the root of patient behaviours. There is now good recognition of the ability of behavioural change theories to encourage patients to proactively manage their healthcare conditions and there are a number of behavioural change models applied in healthcare, including:
- The health belief/health action model. This model suggests that a patient’s belief in a personal threat together with their belief in the effectiveness of behaviour will predict the likelihood of that behaviour. For example, a cardiovascular patient may have certain beliefs about whether it is safe for them to exercise with such a condition.
- The health action process approach. This is a framework of motivation and volition constructs that are assumed to explain and predict individual changes in health behaviours such as quitting smoking, improving physical activity levels or dietary behaviours. This approach suggests that the adoption, initiation, and maintenance of health behaviours should be conceived of as a structured process including a motivation phase and a volition phase.
- The I-Change model. This model assumes at least three phases in the behavioural change process, these being; awareness, motivation and action. ‘Awareness’ is the result of accurate knowledge and risk perceptions of the patient about their behaviour. ‘Motivation’ to change behaviour is dependent on a patient's attitude, social influence beliefs and self-efficacy expectations. While ‘Action’ focuses on self-efficacy, action planning and goal setting.
Incorporating a behavioural layer into the adaptive patient solutions mentioned earlier is another dimension of a solution’s ability to understand and respond to individual patient needs. A solution needs to be able to incorporate elements of a behavioural model, be it methods of data capture, behavioural logic or patient messages. There are more than enough sources of expertise in the market from which a suitable approach for specific diseases or populations can be identified.
When developing a solution for a specific disease population, in addition to understanding aspects of patient segmentation like age, disease stage and medication, we need to remember that patients will also be at different behavioural stages. Therefore the response needs to be different for a patient who is in denial of their condition, than for a patient who has accepted their condition and is trying to learn how to better manage themselves. We also need to bear in mind that once patients progress from one behavioural stage to the next, they may revert back to a previous behavioural stage.
For some, trying to also incorporate behavioural change into healthcare solutions might feel like a bridge too far. Perhaps this is seen as a level of complexity too difficult to incorporate on top of everything else. However, when broken down to its constituent parts, behavioural change can be incorporated into an adaptive platform.
Incentivisation
Behavioural change requires a carrot and stick approach (incentives and disincentives) to inducing behaviours. Patients can either work towards pre-defined goal programmes, which might be specific to their condition or more generally around diet and exercise, or they can define their own personalised goals. Patients can then work towards these goals individually or collaboratively.
In terms of patient collaboration, the universal growth of social media, software apps and gaming has made the concept and acceptance of collaborative engagement common place. This can either take the form of co-operative engagement, which might include patients working together towards a common goal, or patients working individually or in teams in competition with others.
How then, do we plug incentivisation into goal and lifestyle management? For those patients starting out, they could view other existing patient’s socially shared activities. These patients would perhaps display their own individual goals and achievements within the social environment. Patients looking to get started could then find others dealing with similar personal challenges and this acts as a powerful behavioural nudging mechanism to show what can be achieved. For those patients not wanting to engage within a patient community, they can still do their own thing and could still gain tremendous benefit from seeing other patient’s approaches and achievements.
Patients interested in joining the community, could join existing groups and activities or create their own. For example, individual patient step count activity targets could contribute to a group of patients working together to walk the equivalent of Paris to Rome (titled ‘The Paris to Rome Challenge’), a distance of approximately 1,400 kilometres or 1,750,000 steps. Twenty-four patients working together and walking 2,500 steps per day could achieve this target in a month. This kind of social engagement could take any form, be it exercise or diet.
These challenges can also be turned into competitive events, with teams perhaps competing against each other to achieve different targets, for example, first team to arrive, single longest activity in a day and more. We hear a lot about gamification and this is too often associated with onscreen computer games. However, this approach to social engagement offers the ability to build collaborative events amongst patients and provides a very effective form of gaming challenge, in fact this is a growing sector within the traditional gaming market.
You may have noticed that I have spoken about the activities and challenges, but not once mentioned reward. It is important to recognise that providing patients the right kind of support and encouragement to work towards important healthy targets is fundamental to patient engagement. We can then build on this and explore new and appropriate ways to remunerative and financial incentive. This could take the form of a reward points or voucher based system that patients can spend in specified areas.
With any incentive system with monetary value, it will be important to build checks into the system to prevent fraudulent activities. This could simply be a case of linking achievement to clinical outcome, for example a reduction in BMI, possibly requiring verification by a healthcare professional. Although the mechanics of a reward system can be universal.
There is a real opportunity here for the pharmaceutical industry to consider a completely new approach to drug pricing. Programmes like these can provide pharma companies anonymous real world data on patient adherence to their medication and self-management. This provides the opportunity to link reward to drug price by offering discounts to patients who achieve longer term adherence and agreed clinical and health improvements. This discount could go directly to patients who pay for their drug products in some countries or feed through to health insurance premiums in other countries.
The challenges facing healthcare are enormous and as well as joining up concepts, we have to think about bigger changes if we are to respond quickly enough to this crisis. This need is driving a real desire for change with all stakeholders and presenting innovative and revolutionary approaches is well received by regulators, healthcare providers and payers alike.
Mark Brincat