Inner cooling reduces heart and brain damage after MI, cardiac arrest and stroke
Harry Wood, Editor, MTB Europe
11 December 2011
The Philips Inner Cool RTx system provides a rapid method of cooling the body from the inside. The RTx system cools or warms patients with a unique integrated temperature sensor catheter that circulates temperature-controlled fluid within the catheter and adjusts the temperature of the blood near the heart.
It has applications in preventing tissue damage following acute myocardial infarction, heart attack or ischemic stroke using therapeutic hypothermia.
Background to therapeutic hypothermia
References to the use of body cooling for medical purposes go back as far as the Greek physician Hippocrates who recommended the use of snow and ice to treat wounded soldiers. More recently, the first documented clinical use was in the mid twentieth century when ice water baths and open windows in winter were used to treat patients with cancer.
In the last ten years published research has documented the benefits of therapeutic hypothermia in reducing brain damage after cardiac arrest, including studies published in the New England Journal of Medicine in 2002 and endorsed by by the American Heart Association and the International Liaison Committee on Resuscitation in 2005. These recommendations were updated in 2010 and a growing number of hospitals around the world are using hypothermia as standard care for post-cardiac arrest patients.

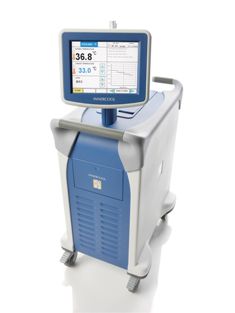
The Philips Inner Cool RTx system with the
Accutrol
temperature modulation catheter
A symposium at Lund University in October this year, supported by Philips, presented an update on hypothermia research in three areas: reducing heart tissue death (infarct volume) in segment elevation myocardial infarction (STEMI), as a therapy for cardiogenic shock and to treat acute ischemic stroke.
Hypothermia to reduce heart tissue damage in acute myocardial infarction
Prof David Erlinge head of cardiology at Lund University, Sweden, presented an overview of recent research on hypothermia as a treatment for acute myocardial infarction, which is the leading cause of mortality in the developed world. A large number of different therapies have been tried without success. Current methods of treatment for acute myocardial infarction focus on returning blood supply to the affected heart tissue as soon as possible to reduce tissue death. However, as the blood returns to the blood vessels of the affected heart area seven times faster than baseline circulation, the patient can suffer reperfusion injury when the vessels are opened. This can lead to additional myocardial damage, secondary complications or even death.
The "Catch 22" problem, said Prof Erlinge, is that reperfusion injury happens during the first 5-10 minutes after AMI and any drug given cannot reach the area of the heart affected before reperfusion. Any drug arriving only a few minutes after reperfusion has no effect.
A pilot study by Lund University published in 2010 (RAPIDMI-ICE), showed that induction of mild hypothermia (< 35°C body temperature) in STEMI patients prior to performing angioplasty can save 38% of heart tissue compared to without hypothermia. The study also indicated that it is safe to induce hypothermia via a combination of cold saline and endovascular cooling (using the Inner Cool closed-loop catheter cooling system) in awake STEMI patients prior to the angioplasty without delaying the time to reperfusion. RAPIDMI-ICE provided the first body of data to demonstrate the existence of cardiac reperfusion injury in humans.
The study concluded that:
- hypothermia should be induced as soon as possible during ischemia;
- hypothermia must be induced before reperfusion;
- an infusion of cold saline alone does not reduce infarct size;
- even hypothermia induced during delayed reperfusion reduces infarct size;
- hypothermia reduces reperfusion injury.
Lund University is now heading a multicentre trial with 120 patients, titled: Efficacy of Endovascular Catheter Cooling Combined with Cold Saline for the Treatment of Acute Myocardial Infarction (CHILL-MI), as a follow up to the pilot study. Early in 2012, CHILL-MI will be enrolling patients at 10 centres in in Sweden, Denmark, Germany and Austria.
Hypothermia to reduce mortality following cardiogenic shock
Dr Matthias Götberg, Department of Cardiology, Lund University presented the outcomes of a preclinical study on hypothermia as a therapy for cardiogenic shock (using pigs).
Cardiogenic shock can result from a weakened heart not being able to pump sufficient blood around the body. It is often caused by a severe heart attack and can be fatal if not treated immediately — it is the most common cause of death following a heart attack. The reduced blood supply leads to a downward spiral of cardiac function, ending with metabolic acidosis and death. The symptoms include hypotension (low blood pressure), altered mental state, low urine output, metabolic acidosis and pulmonary oedema (fluid in lungs).
Pre-clinical and clinical studies have indicated that mild vascular hypothermia can stabilise circulation and improve outcomes. In the study by researchers from the Department of Coronary Heart Disease at Skane University Hospital, Lund University, it was found that inducing mild therapeutic hypothermia using the Inner Cool system resulted in all the pigs surviving compared to a 63% mortality with normothermia (no cooling). Hypothermia reduced the force of circulation following reperfusion, reduced acid imbalance, improved blood pressure and reduced metabolic demand. The findings were published in Resuscitation in 2010.
Hypothermia to treat ischemic stroke
Professor Dr Eric Schmutzhard of the Department of Neurology, Innsbruck University, Austria, discussed current research on using mild hypothermia during ischemic stroke to limit damage to the brain.
In industrialised countries stroke is the second most frequent cause of death and one of the most frequent causes of permanent disability. In the EU there are about 1.5 million new cases of stroke per year and this is expected to increase because of increased life expectancy and improved medical care.
The current options for treating stroke in the acute phase are limited. For ischemic stroke the most efficacious therapy is thrombolysis with a recombinant tissue plasminogen activator (rt-PA) within 4-5 hours after the first signs of ischemic stroke (ESO guidelines). However, there are limitations due to the limited window of opportunity, contraindications (where anticoagulation is not suitable eg preceding severe trauma or surgery), potential reperfusion damage and haemorrhagic complications. Only 5-15% of ischemic stroke patients are treated with rt-PA and only a part of this group clearly benefits from the treatment.
For patients with intracerebral hemorrhage treatment options are also unsatisfactory and neurosurgical intervention does not improve outcome. Morbidity and mortality are high — fatality rates in patients with a large hemorrhage (>30cc) are 90% and virtually none of the surviving patients improve to be able to integrate socially.
In patients with focal acute cerebral ischemia an "ischemic core" of the brain is irreversibly damaged within a short time due to reduction in perfusion following hemorrhage of blood vessels. However, an area of the brain around this core, called the penumbra, has been the target of therapeutic management for preventing further damage.
Therapeutic hypothermia is one of the most studied and effective means of neuroprotection and has been shown to prevent reperfusion injuries that occur after circulation returns to the brain. Injuries can occur well after circulation is restored due to various inflammatory immune responses that occur during reperfusion. Hypothermia has been shown to help minimise the harmful effects of these responses.
A systematic review of the therapeutic aspects of hypothermia in experimental ischemic stroke showed a reduction in infarct volume of 44%. This surpassed all other experimental stroke therapies.
Studies in the US have sought to develop a protocol to safely treat acute stroke patients with endovascular hypothermia. The protocol developed in the ICTuS trial (2005) included a proactive anti-shivering regimen, where researchers were able to induce, maintain and reverse mild hypothermia in conscious stroke victims without the need for paralysis and mechanical ventilation to control shivering.
In 2010 the ICTuS-L trial (Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke) was set up to validate the protocol by evaluating 59 patients. The procedure was well tolerated, with minimal shivering and no rebound hypothermia. It found surface cooling to be less optimal than endovascular cooling that enables rapid achievement of target temperature and precise temperature control.
A further multicentre study is now underway in the US and Europe involving 400 patients — the largest study of its type so far. The ICTuS 2/3 trial is evaluating the benefits of therapeutic hypothermia during stroke to prevent further brain injury or to limit damage.
Regulatory approval
Therapeutic hypothermia is approved in Europe as a therapy to reduce infarct volume heart tissue death for STEMI patients, and to provide neuroprotective therapy in ischemic stroke patients. It is not yet approved for these indications in the US. It is not yet approved as a therapy for cardiogenic shock in Europe or the US.
The Philips Inner Cool system
The Inner Cool RTx can rapidly raise or lower body temperature of certain non-paralysed, awake patients in an intensive care setting. It cools or warms patients with the integrated temperature sensor catheter, which is placed below the heart via the femoral vein, and circulates temperature-controlled fluid within the catheter.
The closed-loop system has the advantage that it can rapidly modulate whole body temperature without introducing fluid into the bloodstream. The average cooling rates for the system are 4-5C/hr and the average warming rates are 2-3C/hr. The rapid cooling reduces the time during which drugs are required to manage shivering in the patient.
The system includes a programmable console with a touch panel screen and an intuitive user interface. The compact console can easily be wheeled around to where it is needed (see images above).
Below is an animation showing how the cooling system acts on the bloodstream: