Tarilian Laser Technologies achieves greatest technological advance in blood pressure measurement for 130 years
Harry Wood, Editor, MTB Europe
A summary
of this article
can be republished using
the press release >
7 December 2011
Welwyn Garden City, UK. - Tarilian Laser Technologies (TLT) has invented a completely novel method of measuring blood pressure based on an optoelectronic sensor. The sensor outperforms the current “gold standard” for measuring blood pressure — the mercury sphygmomanometer — and effectively makes the older technology obsolete.
The accuracy, data collection, size and ease of use of the sensor are likely to have a profound effect on blood pressure measurement in the clinical setting, medical research, and also home healthcare. TLT's next-generation Sapphire sensor system allows direct measurement of systolic and diastolic blood pressure without a cuff — and all within seconds.
A further advantage is that the sensor doesn't just measure blood pressure, it generates a continuous beat-to-beat blood pressure measurement, pulse wave velocity, as well as other 'haemodynamic' data, which in combination enable it to give a more complete vascular assessment than any other blood pressure device. This is a major achievement as it gives information about the health of the whole vascular system including the heart — all from a peripheral sensor. This will give clinicians a much improved toolset for monitoring, diagnosing and treating patients suffering from a wide range of diseases.
These abilities alone make it a world-beating technology, but what makes the sensor even more groundbreaking is its ability to measure blood pressure on virtually any part of the body without exerting pressure and with no energy entering the body. This creates additional unique applications:
- blood pressure measurement of the eye without putting pressure on the eye;
- foetal heart monitor;
- health and blood pressure of the arteries in the leg;
- health and blood pressure of the neck arteries (the carotid); and
- highly accurate miniature sports biometric devices.

The actual core TLT
optoelectronic sensor base.
This is all that is required to make the
BP measurement itself. No cuff is needed.
Dr Sandeep Shah, CEO of TLT, said, “This an exciting time for us. The TLT company was founded and developed in Hertfordshire, UK and within a short period of time we have broken the barrier on sensor capabilities with our novel optical technology. The TLT sensor, because of its power, simply makes other technologies in medical biometrics obsolete, so we see the potential to have our sensor in every blood pressure device — which is a potential worldwide market expanding to a projected 100 million devices a year — projecting TLT into a billion plus dollar company within a short period of time.
“The technology is very scalable and affordable; and indeed can compete extremely well with current economic costs within the BPM sector, and yet it offers unique and powerful advantages over all other technologies. These features simply do not exist with any other technology and this makes TLT's sensor a disruptive technology that has both redefined the state of the art of blood pressure monitoring and one that has created a paradigm shift in cardiovascular medicine.
"It has even been suggested that the ability of the TLT sensor to collect such a rich set of data on the cardiovascular system has opened up a whole new area of physiology. Furthermore we have created a unique proprietary production process of this optical sensor to manufacture large volumes, making us ready to capture this market with ease.”
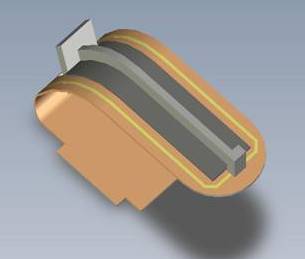
Schematic of the overall TLT core sensor
Cut-through TLT sensor within outside casing
Dr Art Tucker, Principal Clinical Scientist and Vascular Researcher at St Bartholomew’s Hospital, London, said, “The TLT sensor is a highly novel promising new technology that will have a large and positive impact in this field. It is an exciting and powerful new development in vascular science and has created a new state of the art in blood pressure measurement. It will no doubt be of great value both in hospitals and primary care as well as at home for the consumer, and offers a new paradigm in vascular biometrics.”
Dr David Jefferys, ex Chief Executive and Director of the UK Medical Devices Agency, current President of The Organisation for Professionals in Regulatory Affairs (TOPRA), said, "Blood pressure is a critical biometric measurement in medicine and has ubiquitous utility in all areas of medicine and also in research, including pharmaceutical trials. There has been significant controversy and debate about the current BPM technologies — ausculatory, oscillometry and tonometry, especially. A lot of this debate has centred around the inaccuracies and the poor reliability of these systems, which has led to a series of regulatory concerns and investigations by the MHRA, US FDA and also AAMI.”
"These concerns are of serious nature,” Dr Jefferys continued, “not just relating to causative morbidity but also mortality. Indeed, of particular note are the concerns of poor performance of these systems in sub-populations eg the elderly, children and also in pregnancy. A series of recent papers has highlighted technical performance issues with these older systems and also the limitations of their accuracy and reliability. Hence, there is a clear need for a better and more accurate, more robust, versatile technology to be made available within this critical field of medicine — not just from the view of experts but also from a general patient and consumer perspective.
“Simply put, the TLT Technology offers a significant competitive advantage to all others that are in the market. Furthermore, TLT’s Sapphire cuffless sensor promises to deliver a sophisticated and elegant solution to future demands within this sector. The TLT Sapphire sensor development has created a pioneering platform from which further technological advances in haemodynamic profiling may be realised and thus improve the management of an array of medical conditions."
Development of the TLT sensor
Dr Shah, CEO of TLT, is a practising medical doctor, medical technology expert and has also served six years as medical director and board member of a large medical device company. He has a long history of research and clinical practice in hypertension and is a founding member of the IPAC/ Medical Device Committee of NICE.
His interest in hypertension led to him becoming involved in international committees on blood pressure measurement. From what he learnt on these he was stunned by the problems associated with the current technology, so decided to find a better technology. In 2005, he and Nita Shah, Director of TLT, completed a research programme in which they discovered that, by using light in a unique technique developed by them, blood pressure could be determined without any penetration of light into the body. They set up TLT in 2006 to develop the technology further.
Following completion of the initial research programme the company filed a series of patents and set up an international R&D team to progresses with the development of the unique sensor technology. TLT has already successfully completed its international FDA and MHRA pivotal regulatory clinical trial, working with global experts in clinical medicine and clinical trials, including from Barts Hospital, London and the William Harvey Institute, London.
The results of this trial are spectacular and TLT has clearly broken the barrier of accuracy that the current oscillometric system (which uses a pressure cuff) of blood pressure measurement is plagued with. The TLT trial achieved a new world standard with a standard deviation of only 2.8 mm Hg and below. This is a ground-breaking result and has catapulted TLT into the superior technology position. Furthermore, internal research at TLT on over 7,000 datasets has shown that TLT can even achieve standard deviations of less than 1mm Hg — equivalent to a new global gold standard for blood pressure measurement.
Dr Shah said, “Within only 17 months of incorporation, TLT achieved significant international recognition and won a major global Medtech Industry award — the 2007 US LSA Technology Showcase award. TLT is the only non-US company to have won this highly prestigious award. In addition, in February 2009, TLT was awarded two major UK Government grants for the development of two new medical applications of its sensor technology: foetal heart rate monitoring and peripheral vascular disease.
“It is highly unusual to be awarded two major grants upon first application and within a concurrent time frame; and this signifies TLT’s successful technology and intellectual property base. TLT’s technology has received a very high level of expert external validation, endorsement including by the UK Government and the Patent Office as a result of the grant process and TLT now enjoys significant recognition of the huge potential of its sensor platform.”
TLT is now completing the manufacturing scale-up and translation programme for its first product — a highly innovative consumer blood pressure device that is cuff-based and has unique features including superior accuracy and ease of use. TLT expects that its Homecare division will enter significant sales revenue by 2012. It has already secured advanced sales interest in this device, with first year volumes expected to reach about 1 million units.
In January 2011 TLT began what was planned to be a two-year project to develop a completely cuffless system based on the sensor — under the TLT Sapphire Programme. However, the company achieved the objectives to perfect and complete the system in only five months. Dr Shah commented, “I had never envisaged that we would achieve this in the record time that we have done. This is a testament to our determination, careful planning and the skills of our talented team — but also importantly to the unique power of the TLT technology. Indeed we believe that we have only unlocked about 5 to 7% of the true potential of the technology and its signal.”
TLT is currently pursuing international corporate development partnerships for further commercialisation of this cuffless sensor technology and has entered discussions with selected key global technology corporations.
Dr Shah added, “At present we are completing our development of our Sapphire pre-manufacturing and clinical prototype which will be a ubiquitous module platform that will not just deliver an instant and continuous BPM anywhere in the body but can also then be incorporated into a mobile phone, laptop, mouse, pen, ring, etc. Hence this delta prototype will then be the platform from which we can work with, for example, to develop the sensor into a cellphone infrastructure within six months. We have already begun building partnerships with major industry conglomerates including in the medtech and cellular phone sectors.”
Problems with current methods of BPM
The most common method of blood pressure measurement dates back to the 19th century. Although the method of indirect measurement of blood pressure was developed earlier, the first practical device, Von Basch's sphygmomanometer, was invented in 1881. Since then there have been gradual improvements in the technique, called oscillometry, but the basic principal — of compressing the arm and measuring the pressure that impedes pulsation of the blood (systolic pressure) and taking a second measurement at the point the pulsation returns after releasing the compression (diastolic pressure) — has not changed. Oscillometry was not commercialised until the 1980s and has poor patent protection. It is unreliable, inaccurate and difficult to use. Many devices on the market are unvalidated and have unacceptable levels of inaccuracy.
Other technologies appeared in the late 1990s and early 2000s but these also suffer from inaccuracies. Tonometry is very sensitive to the positioning of the sensor (and hence is fiddly and cumbersome to use), suffers from great variability in readings and uses a 'surrogate' measurement to derive blood pressure. It also requires frequent calibration with oscillometric systems to ensure accuracy. In addition, there are difficulties in measuring blood pressure in certain patient groups: the elderly, patients with arrhythmias, obese patients, children and pregnant women.
Hypertension experts have long reported on the shortcomings of current systems of blood pressure measurement — there is a large amount of evidence published in medical journals — and recently regulatory bodies have also become increasingly concerned about the performance of current devices.
The importance of BPM
Blood pressure is one of the most fundamental measurements taken in healthcare. It is the most common after pulse and temperature, but the most informative to healthcare decision making. Raised blood pressure is the most significant cause of death and disability worldwide (Lopez et al, 2006) and over 2 billion people suffer from high blood pressure.
The monitoring of blood pressure has strong applications in many clinical fields including in stroke prevention, obstetrics, neurology, vascular medicine and diabetes.
The global market for BPM
The potential market for the sensor for blood pressure measurement (BPM) alone is staggering. The global overall hospital BPM market alone is worth over $24 billion and the home BPM market is worth $6bn at retail. However, two factors are likely to see a large rise in blood pressure measurement in the home. The growing costs of healthcare worldwide, the aging population and increase in chronic diseases are shifting the healthcare focus to care in the home and preventative care, so more people will be taking blood pressure on a regular basis at home. TLT's miniature sensor will also make it possible to put blood pressure sensing technology into a completely new range of devices, such as pens, computer mice, mobile phones and clothing, so that the measurement of blood pressure could become completely innocuous and ubiquitous, while still achieving a high level of accuracy.
Dr Shah concluded: “I often reflect on what I would like to achieve in my career in medicine and medical technology development, and to have created the world’s first unique optical sensor that accurately generates a carotid and eye BP makes me feel very satisfied and proud. The benefit to patients and also to the healthy to prevent disease is huge, and of course the associated commercial opportunity is also huge — into the billions of dollars! I believe that when we scale up our future development we will see an innovation explosion in the applications of our technology. And what's more it makes me proud that we intend to create up to 19 new jobs in the first year of production and rising to over 60 in year three.”
Harry Wood, Editor, MTB Europe.
Further information
Tarilian Laser Technologies: www.tarilian-lasertechnologies.com